prada trial|Prada for breast cancer : 2024-10-07 The primary outcome measure of the PRADA II trial is the change in left ventricular ejection fraction (LVEF) by CMR from baseline to 18 months. Secondary . Meer over de advertentie Code voor aanvraag: u-15300122001 Merk: audemars piguet Productnaam: Royal Oak Modelnummer: 15300ST.oo.1220ST.01 Materiaal: Roestvrij .
0 · Prada for breast cancer
1 · Prada clinical trial
2 · Prada breast cancer trial
Ter ere van het 30-jarige jubileum van de Royal Oak Offshore komt AP nu met een speciaal horloge, geïnspireerd door de limited edition “End of Days” uit 1999 uit de video hierboven. De nieuwe Royal Oak Offshore .
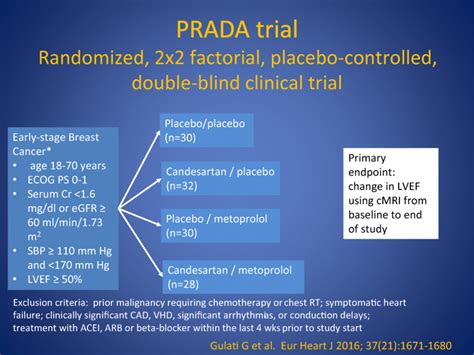
prada trial*******In this 2-year follow-up study of the PRADA trial (Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy), treatment with candesartan . Primary radiotherapy and deep inferior epigastric perforator flap reconstruction for patients with breast cancer requiring mastectomy (PRADA): a multicentre, . Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of . In the PRADA trial (Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy), concomitant treatment with the angiotensin receptor . The primary outcome measure of the PRADA II trial is the change in left ventricular ejection fraction (LVEF) by CMR from baseline to 18 months. Secondary . PRADA II is the first randomized, placebo-controlled study of sacubitril/valsartan in a cardioprotective setting during (neo-)adjuvant breast cancer . The PRADA trial was a randomized, 2×2 factorial, placebo-controlled, double-blind clinical trial conducted at Akershus University Hospital in Norway. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki. The study protocol was reviewed and approved by the Regional Ethics Committee of South .
The primary outcome measure of the PRADA II trial is the change in left ventricular ejection fraction (LVEF) by CMR from baseline to 18 months. Secondary outcomes include change in LV function by global longitudinal strain by CMR and echocardiography and change in circulating cardiac troponin concentrations.
The primary outcome measure of the PRADA II trial is the change in left ventricular ejection fraction (LVEF) by CMR from baseline to 18 months. Secondary outcomes include change in LV function by global longitudinal strain by CMR and echocardiography and change in circulating cardiac troponin concentrations. Results: .
Methods and results: PRevention of cArdiac Dysfunction during Adjuvant breast cancer therapy (PRADA) was a 2 × 2 factorial, placebo-controlled, double-blinded trial of candesartan and metoprolol. Sixty-nine women had valid ECV measurements. ECV fraction, total ECV, and total cellular volume were measured by cardiovascular magnetic .Data from the PRADA trial cannot be publicly shared because of the risk of violating privacy, as regulated by the institutional data protection officer. The PRADA trial was a randomized, 2×2 factorial, placebo-controlled, double-blind clinical trial Clinical Perspective What Is New? • In this 2-year follow-up study of the PRADA trial -prada trialIn the PRADA trial, the investigators followed the . concept of avoiding radiotherapy to the autologous breast reconstruction, thus potentially reducing the risk of fat necrosis, flap fibrosis, and radiotherapy-related flap distortion, with the advantage of a DIEP flap reconstruction that results in less morbidity at
Aadil A Khan, Jennifer E Rusby, Dimitri J Hadjiminas†, Fiona A MacNeill†, on behalf of the PRADA Trial Management Group‡ Summary. BackgroundRadiotherapy before mastectomy and autologous free-flap breast reconstruction can avoid adverse radiation . effects on healthy donor tissues and delays to adjuvant radiotherapy.
In the PRADA trial (Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy), concomitant treatment with the angiotensin receptor blocker candesartan attenuated the reduction in left ventricular ejection fraction (LVEF) in women receiving treatment for breast cancer, whereas the β-blocker metoprolol attenuated the . The PRADA (Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy) trial was a 2 x 2 factorial, randomized controlled trial of candesartan and metoprolol in women with early breast cancer undergoing therapy with anthracyclines with or without trastuzumab. 6 Metoprolol use was not associated with attenuation in LVEF .
The PRADA trial showed that candesartan, but not metoprolol was effective at preserving LVEF among women undergoing chemotherapy for breast cancer. Description: The goal of the trial was to evaluate treatment with a beta-blocker and/or angiotensin-receptor blocker (ARB) among patients with breast cancer undergoing . a, Study design of the PRADO trial.(1) Adjuvant radiotherapy according to patient and physician decisions and (2) according to institute standards. b, Flow chart of the PRADO trial. 1 For one .breast cancer therapy (PRADA) II trial. Methods Study design and objectives PRADA II is a prospective, multicenter, randomized, placebo-controlled, double blinded, parallel group, investigator initiated clinical trial evaluating the eect of sacubitril/valsartan on cardiotoxicity in patients with early breast cancer undergoing treatment with anthra- Methods and results. In a 2 × 2 factorial, randomized, placebo-controlled, double-blind trial, we assigned 130 adult women with early breast cancer and no serious co-morbidity to the angiotensin receptor blocker candesartan cilexetil, the β-blocker metoprolol succinate, or matching placebos in parallel with adjuvant anticancer therapy.prada trial Prada for breast cancer The primary outcome measure of the PRADA II trial is the change in left ventricular ejection fraction (LVEF) by CMR from baseline to 18 months. Secondary outcomes include change in LV function by . The PRADA trial (Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy) investigated whether anthracycline-treated patients with breast cancer were protected from CTRCD with candesartan or metoprolol. 8 The overall decline in left ventricular ejection fraction (LVEF) on cardiac magnetic resonance was only 2.6 .

The PRADA trial was a randomized, 2×2 factorial, placebo-controlled, double-blind clinical trial conducted at Akershus University Hospital in Norway. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki. 120 women with early breast cancer undergoing adjuvant treatment with anthracyclines and/or trastuzumab, were included in the PRADA (PRevention of cArdiac Dysfunction during Adjuvant breast cancer) trial and randomized in a 2x2 factorial fashion to concomitant treatment with candesartan cilexetil, metoprolol succinate or matching .
To our knowledge, few studies of PreMRT followed by breast reconstruction have been conducted, and most used whole-breast CF-RT with a dose of 50 Gy and pedicled flap or implant reconstruction. 18,20-22,41,47-50 The PRADA trial, in which 33 patients underwent preoperative HF-RT (either 40 Gy in 15 fractions or 42.72 Gy in 16 .
Get your International Sales Warranty extended from 2 to 5 years for any .
prada trial|Prada for breast cancer